Study Types
Dr. Vince Clinical Research is a full-service CRO specializing in early phase clinical trials across various designs, therapeutic areas, and drug classes. Most of these studies are performed at our world-class phase 1 research facility, with others performed through strategic partnerships with qualified external sites where DVCR would serve as the contract research organization.
General Studies
First-In-Human
First-in-Human
For over two decades, our team has performed First-in-Human clinical studies for both small and large molecules and vaccines across various therapeutic areas and routes of administration.
SAD/MAD
SAD/MAD
The DVCR team has significant expertise in the conduct of both Single and Multiple Ascending Dose trials. Specifically, Dr. Vince has served as an Investigator on nearly 50 SAD/MAD studies throughout his career.

Drug-Drug Interaction
Drug-Drug Interaction
Drawing from deep experience in drug-drug interaction studies, the DVCR team is exceptionally skilled in the execution of these types of studies, from recruiting and retaining volunteers to procuring and preparing probes and other medications.
Early QT and Thorough QT Cardiac Assessments
Early QT and Thorough QT Cardiac Assessments
DVCR is committed to advancing medicine by leveraging market-leading technology to conduct your Thorough QT or early precision QT study and determine the potential for a medication to affect the cardiac conduction cycle.
PK/PD
Pharmacokinetic/ Pharmacodynamic
We are staffed with a team of highly trained and experienced phlebotomists and other staff who understand the requisite precision and time management skills necessary to conduct a PK/PD clinical trial successfully.

Food Effect
Food Effect
The DVCR team has taken part in many Food Effect studies and possesses the resources and expertise necessary to execute both Food Effect and Fed/Fasted studies effectively.
Proof of Concept (POC)
Proof of Concept (POC)
The experienced medical and research team at DVCR has participated in a large number of Proof-of-Concept trials in a variety of therapeutic areas.
Patient Studies
Patient Studies
By leveraging our robust qualified external site network, we can recruit patient populations needed for Phase I-II in numerous therapeutic indications.

Biosimilars
Biosimilars
Our team has been involved in the conduct of a large number of Biosimilar clinical trials. Further, our pharmacy team has sourced various positive controls for these types of studies.
BA/BE
Bioavailability/Bioequivalence
DVCR supports bioavailability and bioequivalence studies in various dosage forms, including tablets, capsules, inhaled, injectables, topicals, IVs, and others.
Renal Impairment
Renal Impairment
The team at DVCR understands the unique challenges associated with the conduct of renal impairment studies. Our strategic partnership with external sites helps ensure timely recruitment of patients with varying levels of impairment, including severe and those on hemodialysis.
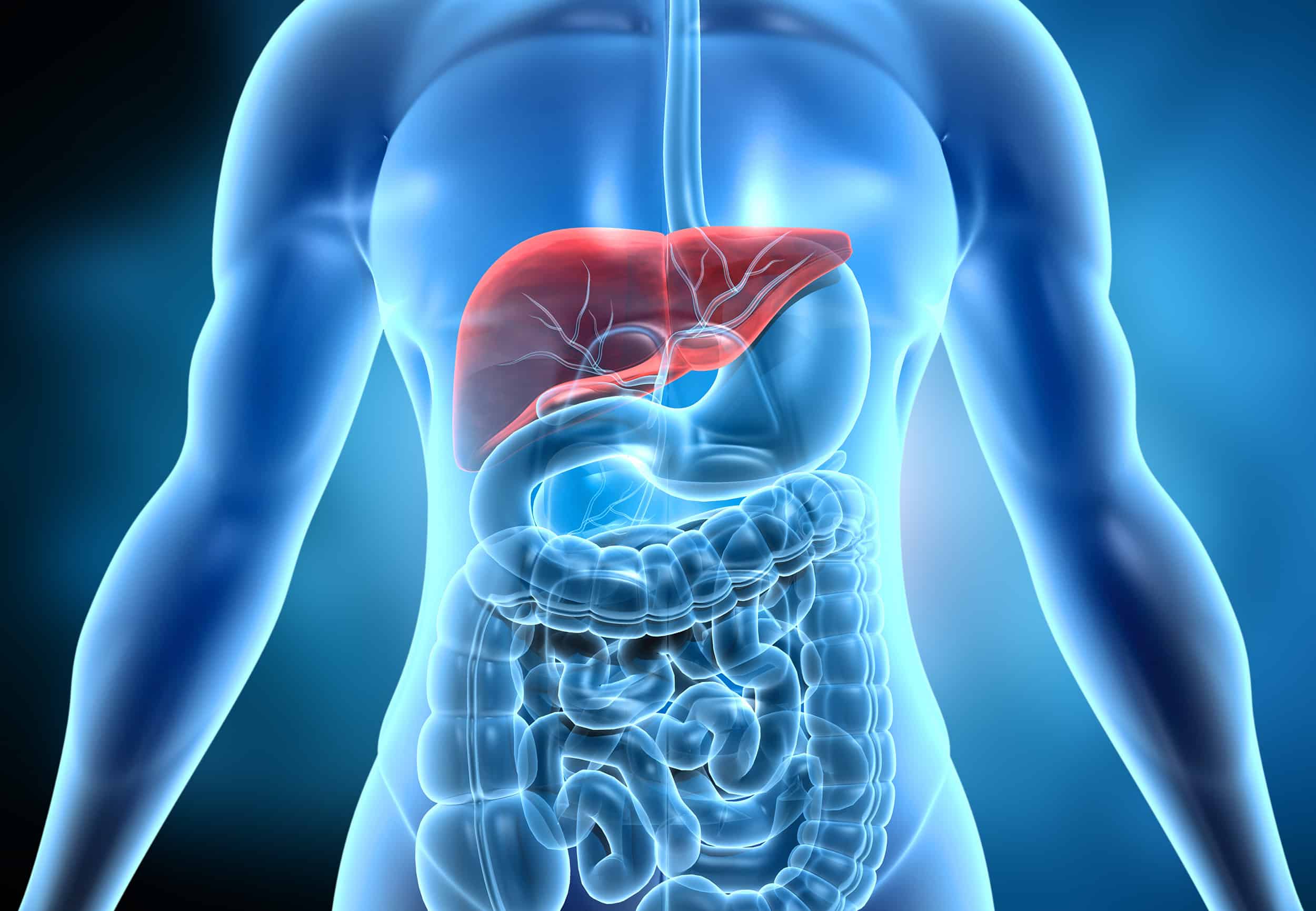
Hepatic Impairment
Hepatic Impairment
DVCR has launched a unique, site-centric approach to successfully manage hepatic impairment studies, with access to mild, moderate, and severely impaired patients.
Dr. Vince Clinical Research is a full-service CRO specializing in early phase clinical trials across various designs, therapeutic areas, and drug classes. Most of these studies are performed at our world-class phase 1 research facility, with others performed through strategic partnerships with qualified external sites where DVCR would serve as the contract research organization.
General Studies

First-In-Human
First-in-Human
For over two decades, our team has performed First-in-Human clinical studies for both small and large molecules and vaccines across various therapeutic areas and routes of administration.
SAD/MAD
SAD/MAD
The DVCR team has significant expertise in the conduct of both Single and Multiple Ascending Dose trials. Specifically, Dr. Vince has served as an Investigator on nearly 50 SAD/MAD studies throughout his career.

Drug-Drug Interaction
Drug-Drug Interaction
Drawing from deep experience in drug-drug interaction studies, the DVCR team is exceptionally skilled in the execution of these types of studies, from recruiting and retaining volunteers to procuring and preparing probes and other medications.

Early QT/TQT
Early QT and TQT Cardiac Assessments
DVCR is committed to advancing medicine by leveraging market-leading technology to conduct your Thorough QT or early precision QT study and determine the potential for a medication to affect the cardiac conduction cycle.
PK/PD
Pharmacokinetic/ Pharmacodynamic
We are staffed with a team of highly trained and experienced phlebotomists and other staff who understand the requisite precision and time management skills necessary to conduct a PK/PD clinical trial successfully.

Food Effect
Food Effect
The DVCR team has taken part in many Food Effect studies, and possesses the resources and expertise necessary to execute both Food Effect and Fed/Fasted studies effectively.
Proof of Concept (POC)
Proof of Concept (POC)
The experienced medical and research team at DVCR has participated in a large number of Proof-of-Concept trials in a variety of therapeutic areas.
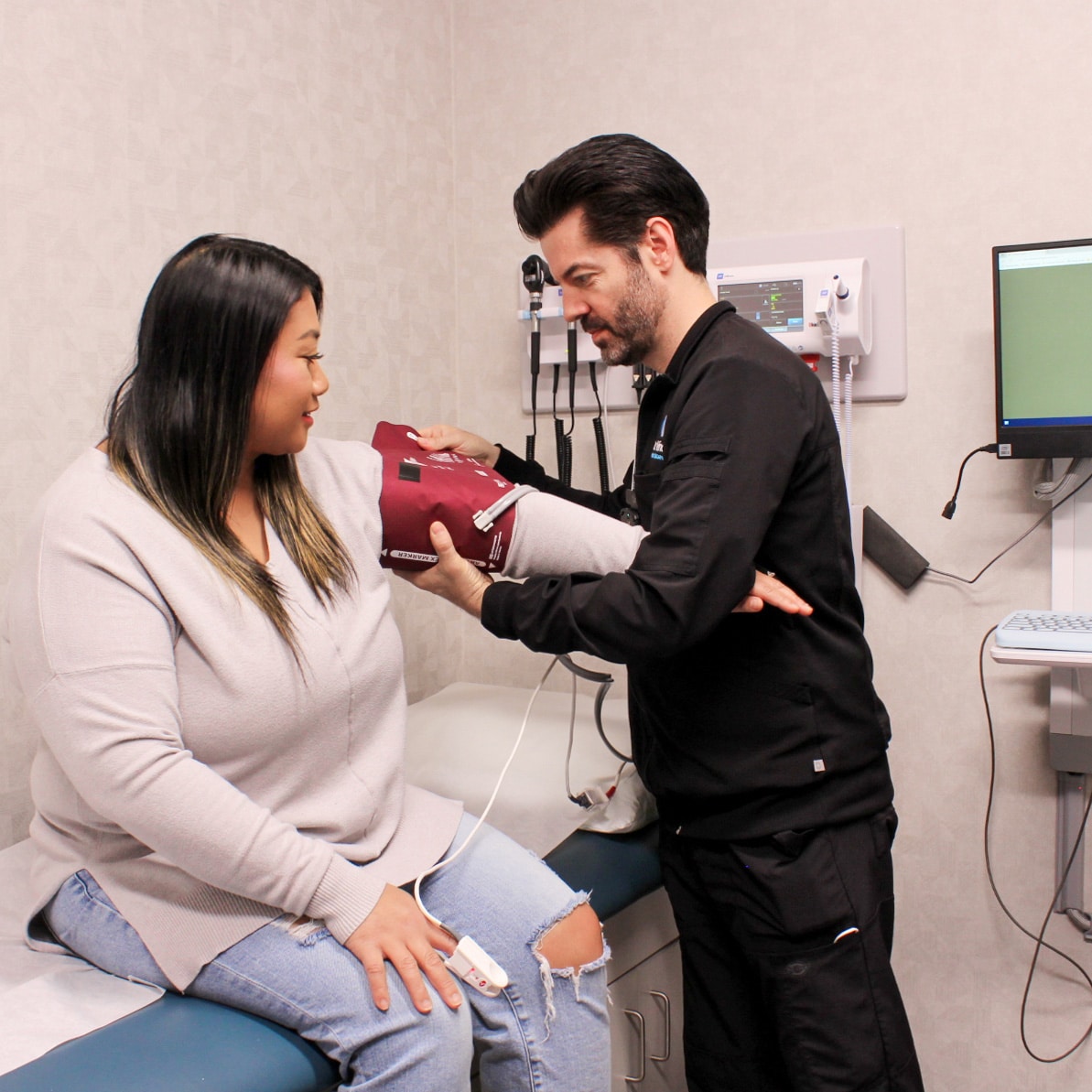
Patient Studies
Patient Studies
By leveraging our robust qualified external site network, we can recruit patient populations needed for Phase I-II in numerous therapeutic indications.

Biosimilars
Biosimilars
Our team has been involved in the conduct of a large number of Biosimilar clinical trials. Further, our pharmacy team has sourced various positive controls for these types of studies.

BA/BE
Bioavailability/ Bioequivalence
DVCR supports bioavailability and bioequivalence studies in various dosage forms, including tablets, capsules, inhaled, injectables, topicals, IVs, and others.
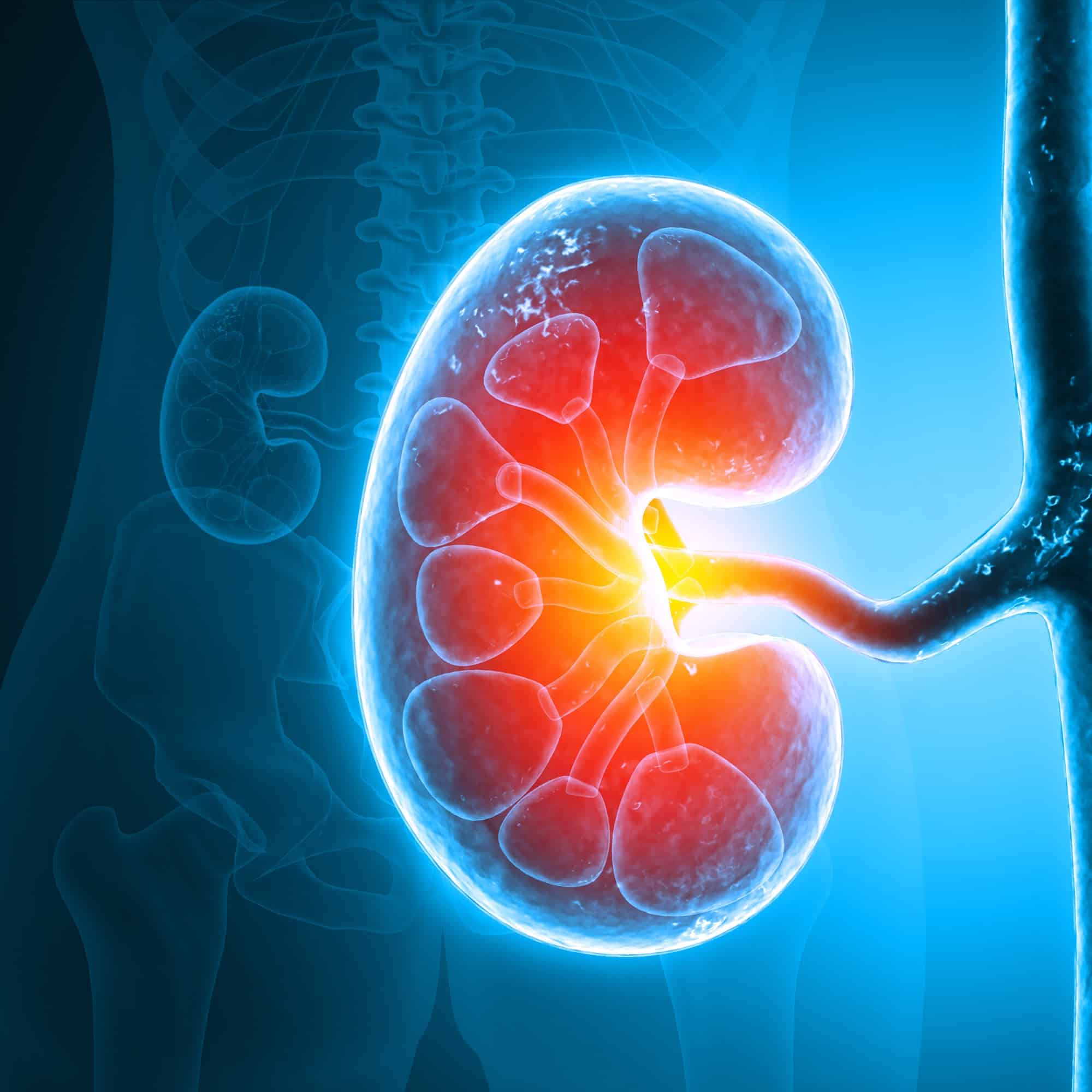
Renal Impairment
Renal Impairment
The team at DVCR understands the unique challenges associated with the conduct of renal impairment studies. Our strategic partnership with external sites helps ensure timely recruitment of patients with varying levels of impairment.
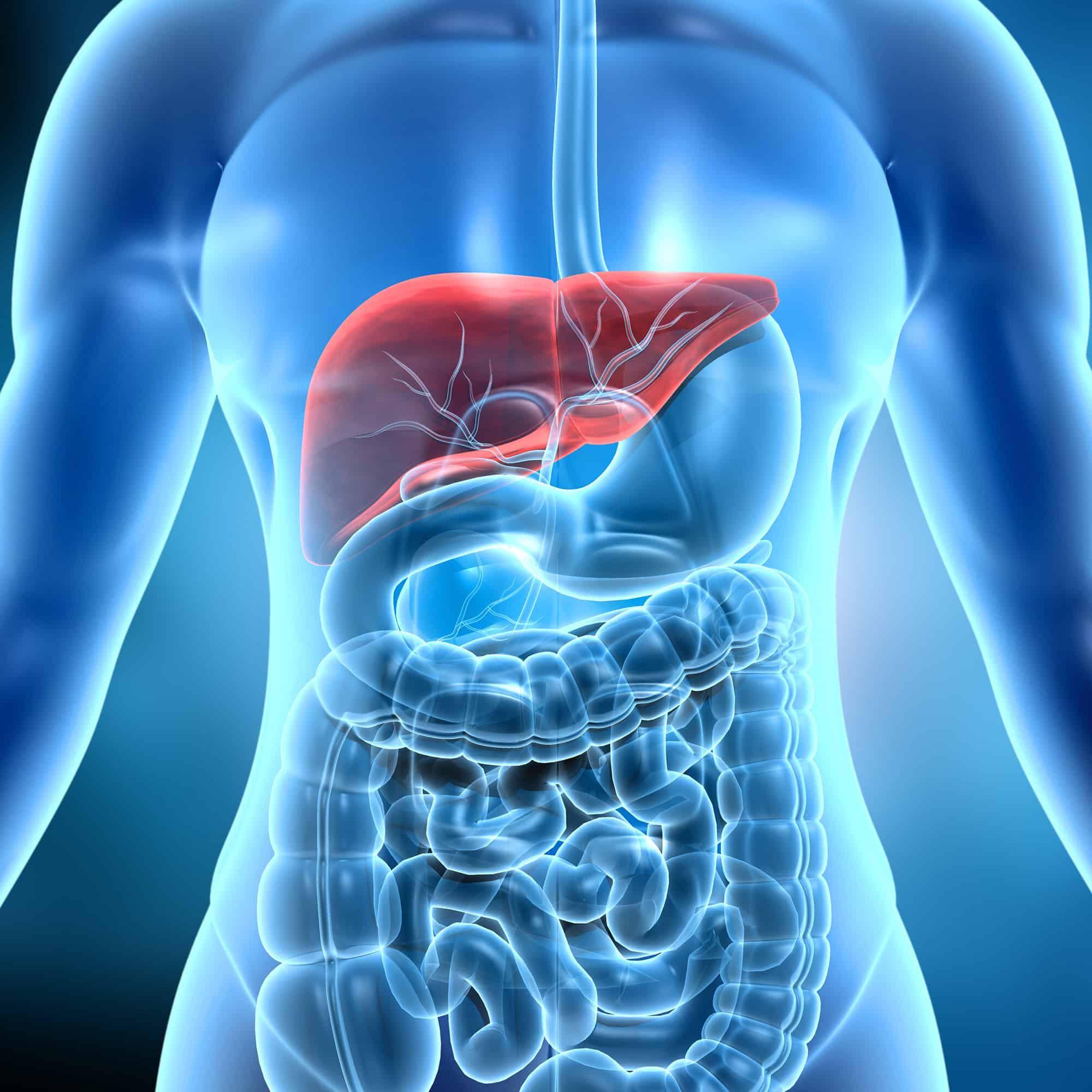
Hepatic Impairment
Hepatic Impairment
DVCR has launched a unique, site-centric approach to successfully manage hepatic impairment studies, with access to mild, moderate, and severely impaired patients.
Dr. Vince Clinical Research is a full-service CRO specializing in early phase clinical trials across various designs, therapeutic areas, and drug classes. Most of these studies are performed at our world-class phase 1 research facility, with others performed through strategic partnerships with qualified external sites where DVCR would serve as the contract research organization.
General Studies

First-In-Human
First-in-Human
For over two decades, our team has performed First-in-Human clinical studies for both small and large molecules and vaccines across various therapeutic areas and routes of administration.

SAD/MAD
SAD/MAD
The DVCR team has significant expertise in the conduct of both Single and Multiple Ascending Dose trials. Specifically, Dr. Vince has served as an Investigator on nearly 50 SAD/MAD studies throughout his career.

Drug-Drug Interaction
Drug-Drug Interaction
Drawing from deep experience in drug-drug interaction studies, the DVCR team is exceptionally skilled in the execution of these types of studies, from recruiting and retaining volunteers to procuring and preparing probes and other medications.
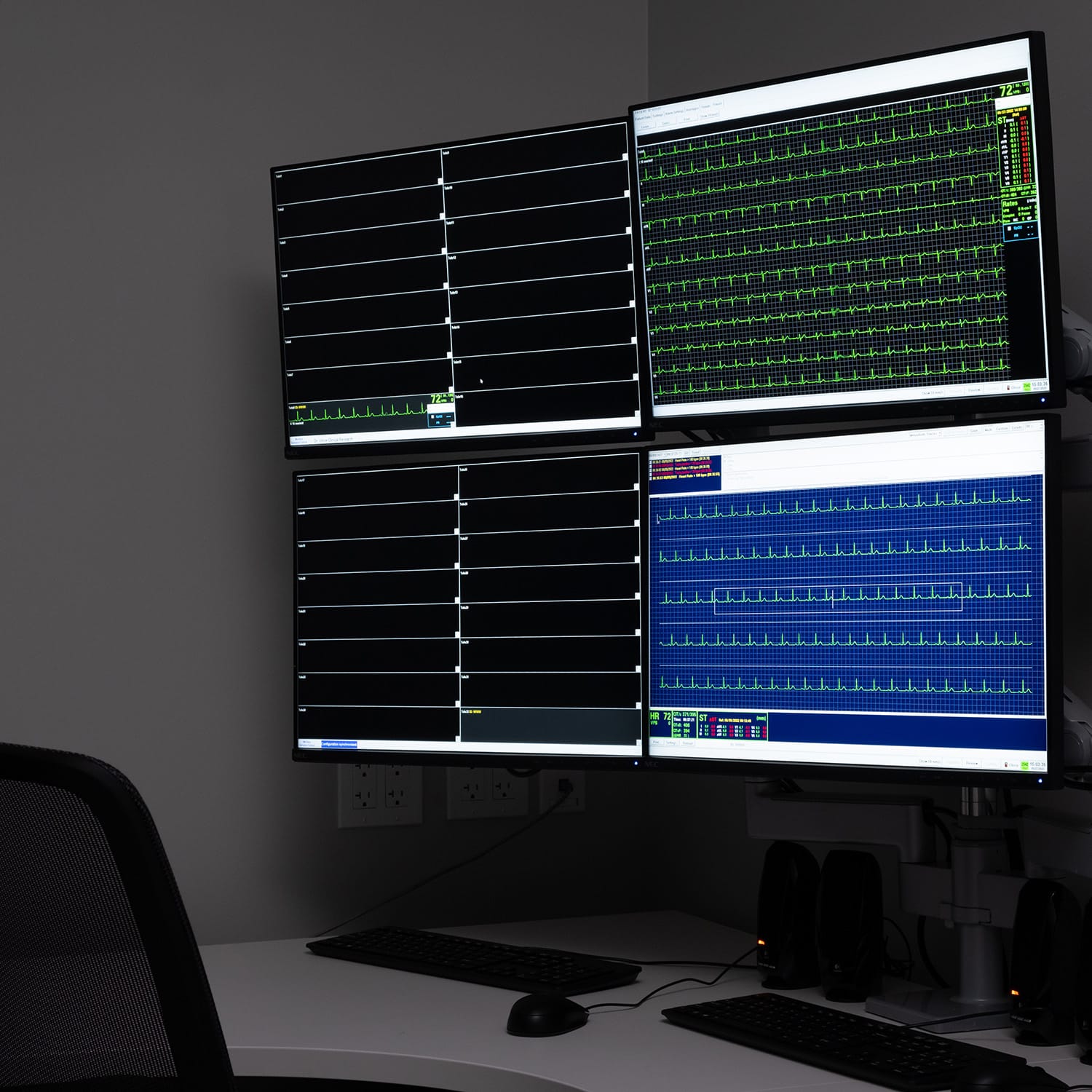
Early QT/TQT Assessments
Early QT/TQT Assessments
DVCR is committed to advancing medicine by leveraging market-leading technology to conduct your Thorough QT or early precision QT study and determine the potential for a medication to affect the cardiac conduction cycle.

PK/PD
Pharmacokinetic/ Pharmacodynamic
We are staffed with a team of highly trained and experienced phlebotomists and other staff who understand the requisite precision and time management skills necessary to conduct a PK/PD clinical trial successfully.

Food Effect
Food Effect
The DVCR team has taken part in many Food Effect studies, and possesses the resources and expertise necessary to execute both Food Effect and Fed/Fasted studies effectively.

Proof of Concept (POC)
Proof of Concept (POC)
The experienced medical and research team at DVCR has participated in a large number of Proof-of-Concept trials in a variety of therapeutic areas.

Patient Studies
Patient Studies
By leveraging our robust qualified external site network, we can recruit patient populations needed for Phase I-II in numerous therapeutic indications.

Biosimilars
Biosimilars
Our team has been involved in the conduct of a large number of Biosimilar clinical trials. Further, our pharmacy team has sourced various positive controls for these types of studies.

BA/BE
Bioavailability/ Bioequivalence
DVCR supports bioavailability and bioequivalence studies in various dosage forms, including tablets, capsules, inhaled, injectables, topicals, IVs, and others.

Renal Impairment
Renal Impairment
The team at DVCR understands the unique challenges associated with the conduct of renal impairment studies. Our strategic partnership with external sites helps ensure timely recruitment of patients with varying levels of impairment.

Hepatic Impairment
Hepatic Impairment
DVCR has launched a unique, site-centric approach to successfully manage hepatic impairment studies, with access to mild, moderate, and severely impaired patients.
Specialized Studies

Human Abuse Potential
Human Abuse Potential
Our team possesses deep industry experience to support the design, planning, and conduct of your Human Abuse Liability (HAL) study in recreational drug users for the following drug classes: stimulants, opioids, sedative-hypnotics, THC/CBD, and hallucinogenics.
Experimental Pain Models
Experimental Pain Models
We conduct a variety of experimental pain model clinical trials (from capsaicin to cold pressor) under the guidance of our Executive Vice President of Scientific Affairs, Lynn R. Webster, MD.

Human Pain Models
Human Pain Models
Human pain models are conducted in volunteers with diseased states undergoing a surgical procedure, most commonly a bunionectomy or third molar extraction.

Tobacco & Nicotine Harm Reduction
Tobacco & Nicotine Harm Reduction
DVCR has significant expertise in the conduct of tobacco/nicotine trials required for PMTA and MRTP FDA submissions. Our leadership team has conducted an average of 10 tobacco/nicotine clinical trials.
Cognitive Testing
Cognitive Testing
DVCR has experience performing numerous cognitive and psychometric tests using various validated scales and platforms. Our full-time Ratings Manager supervises these assessments.
Substance Abuse/Dependance
Substance Abuse/Dependance
Our team has performed a significant number of overnight Phase I-II trials in recreational and substance abuse populations.
Ventilatory Response Model
Ventilatory Response Model
DVCR has the expertise to design studies that can evaluate the effect a drug has on inducing respiratory depression or reversing drug-induced respiratory depression.
As an early phase CRO, Dr. Vince Clinical Research provides a full suite of clinical trial services, conducting clinical pharmacology research and consulting on clinical trial design. Our CRO services include project management, data management, biostatistics and programming, PK/PD analysis, monitoring, medical writing and external site feasibility and management.
Specialized Studies

Human Abuse Potential
Human Abuse Potential
Our team possesses deep industry experience to support the design, planning, and conduct of your Human Abuse Liability (HAL) study in recreational drug users for the following drug classes: stimulants, opioids, sedative-hypnotics, THC/CBD, and hallucinogenics.
Experimental Pain Models
Experimental Pain Models
We conduct a variety of experimental pain model clinical trials (from capsaicin to cold pressor) under the guidance of our Executive Vice President of Scientific Affairs, Lynn R. Webster, MD.

Human Pain Models
Human Pain Models
Human pain models are conducted in volunteers with diseased states undergoing a surgical procedure, most commonly a bunionectomy or third molar extraction.

Tobacco & Nicotine Harm Reduction
Tobacco & Nicotine Harm Reduction
DVCR has significant expertise in the conduct of tobacco/nicotine trials required for PMTA and MRTP FDA submissions. Our leadership team has conducted an average of 10 tobacco/nicotine clinical trials.

Cognitive Testing
Cognitive Testing
DVCR has experience performing numerous cognitive and psychometric tests using various validated scales and platforms. Our full-time Ratings Manager supervises these assessments.

Substance Abuse/Dependance
Substance Abuse/Dependance
Our team has performed a significant number of overnight Phase I-II trials in recreational and substance abuse populations.
Ventilatory Response Model
Ventilatory Response Model
DVCR has the expertise to design studies that can evaluate the effect a drug has on inducing respiratory depression or reversing drug-induced respiratory depression.
As an early phase CRO, Dr. Vince Clinical Research provides a full suite of clinical trial services, conducting clinical pharmacology research and consulting on clinical trial design. Our CRO services include project management, data management, biostatistics and programming, PK/PD analysis, monitoring, medical writing and external site feasibility and management.
Specialized Studies

Human Abuse Potential
Human Abuse Liability (HAL) / Human Abuse Potential (HAP)
Our team possesses deep industry experience to support the design, planning, and conduct of your Human Abuse Liability (HAL) study in recreational drug users for the following drug classes: stimulants, opioids, sedative-hypnotics, THC/CBD, and hallucinogenics.
Experimental Pain Models
Experimental Pain Models
We conduct a variety of experimental pain model clinical trials (from capsaicin to cold pressor) under the guidance of our Executive Vice President of Scientific Affairs, Lynn R. Webster, MD.

Human Pain Models
Human Pain Models
Human pain models are conducted in volunteers with diseased states undergoing a surgical procedure, most commonly a bunionectomy or third molar extraction.

Tobacco & Nicotine Harm Reduction
Tobacco and Nicotine Harm Reduction
DVCR has significant expertise in the conduct of tobacco/nicotine trials required for PMTA and MRTP FDA submissions. Our leadership team has conducted an average of 10 tobacco/nicotine clinical trials.
Cognitive Testing
Cognitive Testing
DVCR has experience performing numerous cognitive and psychometric tests using various validated scales and platforms. Our full-time Ratings Manager supervises these assessments.
Substance Abuse/Dependance
Substance Abuse/Dependance
Our team has performed a significant number of overnight Phase I-II trials in recreational and substance abuse populations.
Ventilatory Response Model
Ventilatory Response Model
DVCR has the expertise to design studies that can evaluate the effect a drug has on inducing respiratory depression or reversing drug-induced respiratory depression.
As an early phase CRO, Dr. Vince Clinical Research provides a full suite of clinical trial services, conducting clinical pharmacology research and consulting on clinical trial design. Our CRO services include project management, data management, biostatistics and programming, PK/PD analysis, monitoring, medical writing and external site feasibility and management.

