Food Effect Studies
Because there is so much variation in diets, the FDA strongly encourages the development of drug formulations that are not affected by food. Unfortunately, developing such formulations is not always possible. Therefore, well-conducted Food Effect studies can inform how, when, and why drugs should or should not be administered with food (1). Evaluating the effect of food on the absorption of an orally administered drug is a key component in improving the safety and efficacy of the product and developing proper instructions for drug administration in relation to food.
Food-drug interactions can manifest in multiple ways. In some instances, co-administration of an orally administered drug with food can increase systemic exposure, which could lead to an increased pharmacologic response that can impact safety and/or efficacy results. Alternatively, administering an oral drug with food can lower the drug’s systemic absorption, therefore reducing its efficacy. Pharmacokinetic studies are conducted to determine the following:
- If, and to what extent if so, food impacts the systemic exposure of the drug.
- Whether administration with food changes the variability of the systemic exposure of the drug.
- If the effect of food varies across meals with different fat or calorie contents.
Understanding the impact of various dosing scenarios is essential for developing the correct dosing instructions for use of the drug. Conducting Food Effect clinical studies early in the drug development process can result in the following conclusions:
- The drug should only be administered under fasted conditions. This means that in subsequent clinical studies, the drug should be administered without food and realistic intervals between drug administration and food intake should be implemented in those trials.
- The drug should be administered with food to alleviate adverse reactions.
- Co-administration of the drug with food could be the only realistic means of ensuring adequate systemic availability for the drug to be effective.
If the observed increase or decrease in the systemic exposure of the drug in the administration with food is not clinically relevant, Sponsors may be able to conduct pivotal safety and efficacy trials without considering food and also include this in the product’s labeling.
DVCR Fed Fasted Study Experience
The Dr. Vince Clinical Research team has taken part in multiple Food Effect studies, and possesses the resources and expertise necessary to quickly execute both pilot and definitive studies as outlined in the recently-updated FDA Guidance Assessing the Effects of Food on Drugs in INDs and NDAs — Clinical Pharmacology Considerations (June 2022). Multiple aspects of our 90-bed clinical pharmacology unit were designed with the conduct of pharmacokinetic studies such as Food Effect clinical trials in mind.
Press Release on DVCR’s involvement in a recent Food Effect Study:
DVCR utilizes a cloud-based eSource system for nearly all aspects of study conduct, including capturing data around meals. Meal sequencing (e.g., fed versus fasted) is built into the system based on how subjects are randomized. Further, meal forms will only be available for those volunteers who are assigned to receive meals for that day, reducing human error.
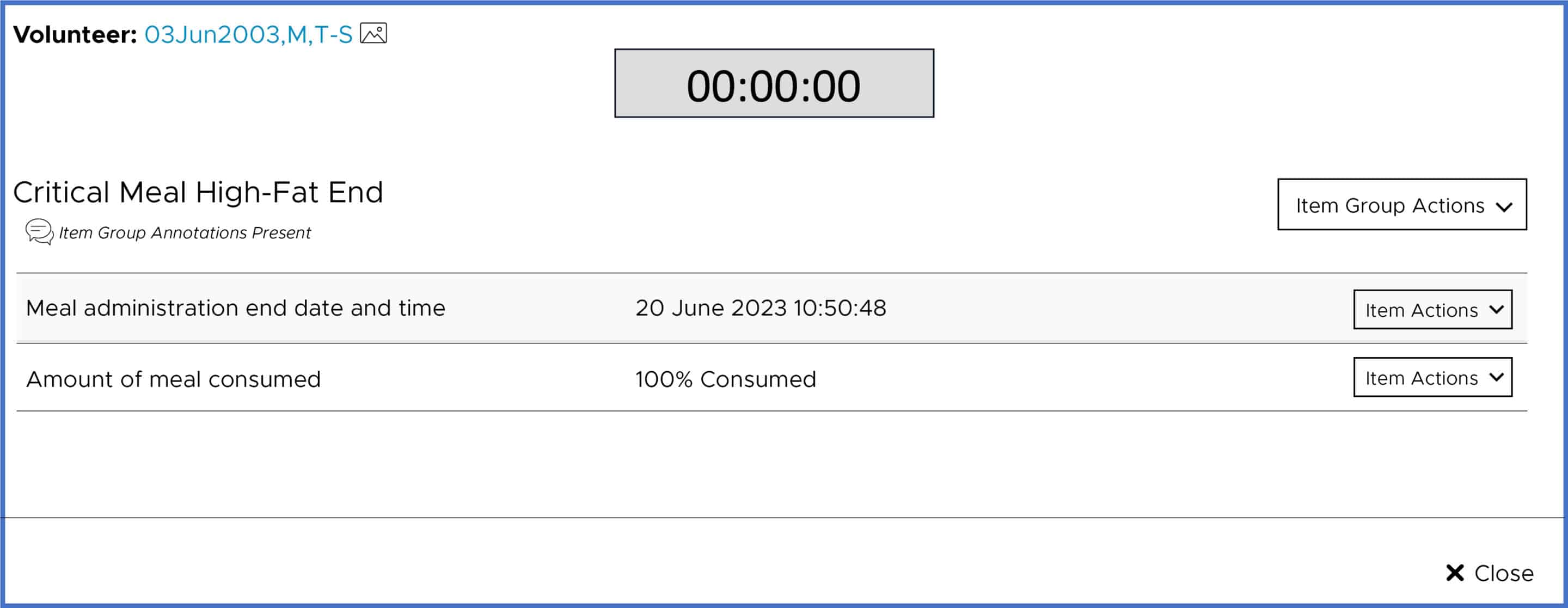
For all critical meals, volunteer wristbands are barcode scanned to capture precise start and stop times, and the percentage consumption amount is recorded in the eSource. The eSource also provides a countdown feature for meal start and stop times.

During all critical meals, a DVCR staff member is assigned to observe the participants in the dining area to ensure compliance with the meal requirements and that the subject is eligible per protocol for dosing. As an added visual indicator for subjects assigned to eat critical meals, meals are dispensed on color-coded trays.
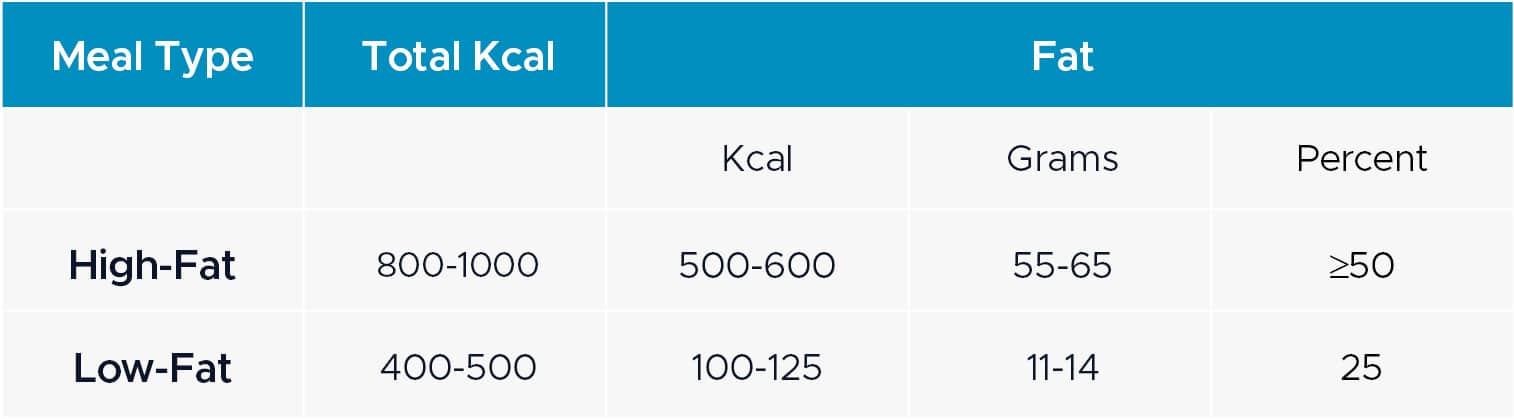
Meals can be prepared in advance or on-site in our fully equipped kitchen for Food Effect trials. For any protocols which outline specific meal composition requirements, DVCR works with a dietician to ensure that the meals meet study-specific specifications. Menus are presented to Sponsors for review and approval prior to study start-up. The FDA guidance document provides the below table for high-fat and low-fat meal compositions:
Read the full FDA Guidance document here.
DVCR Fed Fasted Study Experience
The Dr. Vince Clinical Research team has taken part in multiple Food Effect studies, and possesses the resources and expertise necessary to quickly execute both pilot and definitive studies as outlined in the recently-updated FDA Guidance Assessing the Effects of Food on Drugs in INDs and NDAs — Clinical Pharmacology Considerations (June 2022). Multiple aspects of our 90-bed clinical pharmacology unit were designed with the conduct of pharmacokinetic studies such as Food Effect clinical trials in mind.
Press Release on DVCR’s involvement in a recent Food Effect Study:
DVCR utilizes a cloud-based eSource system for nearly all aspects of study conduct, including capturing data around meals. Meal sequencing (e.g., fed versus fasted) is built into the system based on how subjects are randomized. Further, meal forms will only be available for those volunteers who are assigned to receive meals for that day, reducing human error.
For all critical meals, volunteer wristbands are barcode scanned to capture precise start and stop times, and the percentage consumption amount is recorded in the eSource. The eSource also provides a countdown feature for meal start and stop times.


During all critical meals, a DVCR staff member is assigned to observe the participants in the dining area to ensure compliance with the meal requirements and that the subject is eligible per protocol for dosing. As an added visual indicator for subjects assigned to eat critical meals, meals are dispensed on color-coded trays.

Meals can be prepared in advance or on-site in our fully equipped kitchen for Food Effect trials. For any protocols which outline specific meal composition requirements, DVCR works with a dietician to ensure that the meals meet study-specific specifications. Menus are presented to Sponsors for review and approval prior to study start-up. The FDA guidance document provides the below table for high-fat and low-fat meal compositions:

Read the full FDA Guidance document here.
Food Effect Study Design
Pilot Food Effect Study
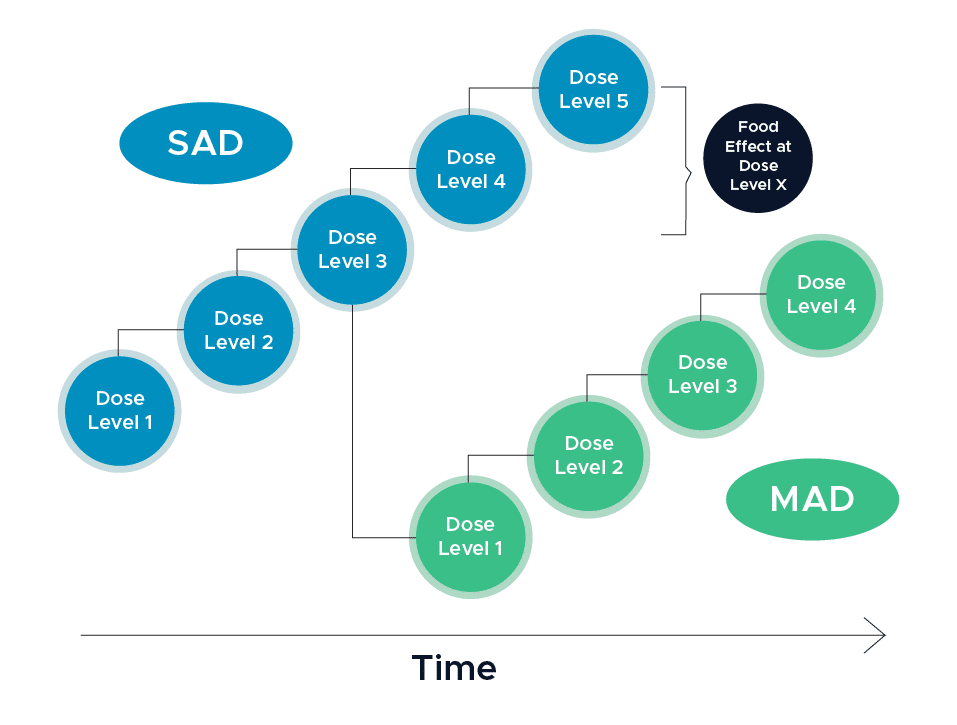
It is recommended that Sponsors conduct a pilot food effect study early in the clinical development, process, such as during a first-in-human study, which will provide a preliminary assessment of the effect of a high-fat meal on the systemic exposure of the drug. “These assessments can be incorporated into the SAD (single ascending dose) arm of the study when dose escalation has reached an anticipated therapeutically relevant level […] Both food and formulation effect assessment should be conducted with a cross-over design to draw meaningful conclusions (2).”
In the event that an important drug-food interaction is observed with a high-fat meal, assessing the interaction with a lower-fat meal could also be useful. Conducting a pilot food effect study can help to inform whether a drug should be administered with food in subsequent clinical trials until a to-be-marketed formulation is available.
Definitive Food Effect Study
For the definitive (pivotal) fed fasted study, the design of the study is typically a randomized, balanced, single-dose, two-treatment, two-period crossover design to assess the effects of food on the orally administered drug product. If two formulations are being assessed in the same study, a three-way crossover study could be considered.
The investigational product should be administered on an empty stomach during one period (fasted) and with the high-fat meal in the other period (fed). In the fasted portion, study volunteers should be administered the drug product with 8 fluid ounces of water following an overnight fast of at least 10 hours. After the dose, participants should not consume any food for at least four hours. Additionally, subjects should be on a water restriction for the hours proceeding and following the dose; additional water is permitted as requested outside of these windows. Throughout the study, subjects will receive standardized meals scheduled at the same time.
In the fed portion, study participants should begin eating a meal 30 minutes prior to product administration following an overnight fast of at least 10 hours. The investigational product should be administered with 8 fluid ounces of water. As with the fasted group, subjects in the fed group should be on a water restriction in the hour preceding and following dose administration. Also, no additional food is allowed for at least 4 hours after the dose.
It is recommended that a washout period of three to five elimination half-lives separate the two treatments (1). However, for drugs with long elimination half-lives, a single-dose, parallel design may be more appropriate. In this instance, separate subjects should be used for each treatment group, and the volunteers in each group should also be of similar demographics.
Unless the investigational product is being developed for an indication specific to one sex, or there would be safety concerns precluding the enrollment of one of the sexes, then the Sponsor should enroll both male and female volunteers. Therefore, working with a clinical site that can effectively enroll both sexes into the food effect trial is important.
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Reach Out Today
If you would like to receive more information regarding how our CRO can be leveraged for your upcoming fed fasted clinical trial, please contact us.
It is recommended that a washout period of three to five elimination half-lives separate the two treatments (1). However, for drugs with long elimination half-lives, a single-dose, parallel design may be more appropriate. In this instance, separate subjects should be used for each treatment group, and the volunteers in each group should also be of similar demographics.
Unless the investigational product is being developed for an indication specific to one sex, or there would be safety concerns precluding the enrollment of one of the sexes, then the Sponsor should enroll both male and female volunteers. Therefore, working with a clinical site that can effectively enroll both sexes into the food effect trial is important.
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Reach Out Today
If you would like to receive more information regarding how our CRO can be leveraged for your upcoming fed fasted clinical trial, please Contact Us.
References
1 U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) . (2022). Assessing the Effects of Food on Drugs in INDs and NDAs — Clinical Pharmacology Considerations Guidance for Industry . Silver Spring, MD; Food and Drug Administration. Retrieved July 3, 2023, from https://www.fda.gov/media/121313/download.
2 Shen, J., Swift, B., Mamelok, R., Pine, S., Sinclair, J., & Attar, M. (2018). Design and conduct considerations for first-in-human trials. Clinical and Translational Science, 12(1), 6–19. https://doi.org/10.1111/cts.12582