Early QT and TQT Cardiac
Assessment Studies
The FDA requires all new chemical entities (NCEs) with systemic exposure to undergo rigorous testing to evaluate the drug’s effect on electrocardiogram (ECG) parameters and potential to prolong the QT interval. The QT interval is defined as the duration of time elapsed between the onset of the “Q wave” and the end of the “T wave” on the ECG tracing (measured in milliseconds). This corresponds to the depolarization and repolarization of the ventricles.
This requirement was implemented as a strategy to reduce the possibility of post-market withdrawals due to arrhythmia and sudden cardiac death, outlined in the International Conference on Harmonization’s (ICH) E14 guidance and usually required the completion of a Thorough QT clinical trial (TQT).
In 2015, the ICH E14 guidance was revised to allow concentration-corrected QT interval analysis to be performed on data generated from First-in-Human (FIH) studies to assess the effect of the NCE on the QT interval. This spurred the development of new methods to collect and assess these effects and gather sufficient data to satisfy regulatory requirements.
Dr. Vince Clinical Research (DVCR) is committed to advancing medicine by leveraging technology as it relates to cardiac rhythm analysis and the potential of medications to affect the cardiac conduction cycle. Be it early precision QT (EPQT) or thorough QT (TQT) analysis, DVCR has the expertise and equipment necessary to conduct these critical trials. Our contemporary approach to cardiac studies enables us to ensure that our pharmaceutical sponsors receive deliverables smoothly and accurately while keeping volunteer safety at the forefront of our efforts.
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
The FDA requires all new chemical entities (NCEs) with systemic exposure to undergo rigorous testing to evaluate the drug’s effect on electrocardiogram (ECG) parameters and potential to prolong the QT interval. The QT interval is defined as the duration of time elapsed between the onset of the “Q wave” and the end of the “T wave” on the ECG tracing (measured in milliseconds). This corresponds to the depolarization and repolarization of the ventricles.
This requirement was implemented as a strategy to reduce the possibility of post-market withdrawals due to arrhythmia and sudden cardiac death, outlined in the International Conference on Harmonization’s (ICH) E14 guidance and usually required the completion of a Thorough QT clinical trial (TQT).
In 2015, the ICH E14 guidance was revised to allow concentration-corrected QT interval analysis to be performed on data generated from First-in-Human (FIH) studies to assess the effect of the NCE on the QT interval. This spurred the development of new methods to collect and assess these effects and gather sufficient data to satisfy regulatory requirements.
Dr. Vince Clinical Research (DVCR) is committed to advancing medicine by leveraging technology as it relates to cardiac rhythm analysis and the potential of medications to affect the cardiac conduction cycle. Be it early precision QT (EPQT) or thorough QT (TQT) analysis, DVCR has the expertise and equipment necessary to conduct these critical trials. Our contemporary approach to cardiac studies enables us to ensure that our pharmaceutical sponsors receive deliverables smoothly and accurately while keeping volunteer safety at the forefront of our efforts.
DVCR’s TQT Experience and Phase I Unit Capabilities

Team of directors and managers with decades of experience working on TQT/EPQT studies
Two clinical research professionals who are certified as Clario “Site Champions”

Dedicated Safety team (ACLS certified)
Market-Leading Technology and Equipment
In order to successfully execute TQT and early QT studies, the clinical pharmacology unit must be well-suited for integration with the latest technology and equipment. Within the primary clinical research unit, DVCR maintains a dedicated cardiac telemetry room equipped with 48 individual monitoring channels on the state-of-the-art Hillrom Surveyor™ cardiac telemetry system. This market-leading equipment represents a significant financial investment as well as a commitment to data quality as it pertains to cardiac monitoring.
The utilization of the central monitoring station and S4 wireless transmitters, which are 12-lead units, means that they can be used for Holter monitoring. Operating on our dedicated Wi-Fi network, the use of our own equipment removes common barriers to data collection such as SD card storage limits and battery failures, both of which are causes of lost data in these types of trials.
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Market-Leading Technology and Equipment
In order to successfully execute TQT and early QT studies, the clinical pharmacology unit must be well-suited for integration with the latest technology and equipment. Within the primary clinical research unit, DVCR maintains a dedicated cardiac telemetry room equipped with 48 individual monitoring channels on the state-of-the-art Hillrom Surveyor™ cardiac telemetry system. This market-leading equipment represents a significant financial investment as well as a commitment to data quality as it pertains to cardiac monitoring.
The utilization of the central monitoring station and S4 wireless transmitters, which are 12-lead units, means that they can be used for Holter monitoring. Operating on our dedicated Wi-Fi network, the use of our own equipment removes common barriers to data collection such as SD card storage limits and battery failures, both of which are causes of lost data in these types of trials.
Key Features of the Hillrom SurveyorTM cardiac telemetry system:
Cardiac data can be archived on-site

Reduced number of electrodes placed on study volunteers improves comfort and leads to better subject retention

Enables data transmission directly to Clario’s cloud-based portal

Site staff can remotely monitor tracing quality and reduce data collection interruptions
The combination of our on-site equipment and Clario’s clinically-proven Early Precision QT methodology optimizes data collection, transmission, and analysis. Waveform interference is minimized by consistently enforcing electronics restrictions within the clinic when ECG data is being collected. In addition, medical procedures are meticulously scheduled around extraction time points in order to make EPQT/TQT trials successful.
If you would like to receive more information regarding how our CRO can be leveraged for your upcoming cardiac assessment study, please contact us.
Key Features of the Hillrom Surveyor™ cardiac telemetry system:

Cardiac data can be archived on-site

Reduced number of electrodes placed on study volunteers improves comfort and leads to better subject retention

Enables data transmission directly to Clario’s cloud-based portal

Site staff can remotely monitor tracing quality and reduce data collection interruptions
The combination of our on-site equipment and Clario’s clinically-proven Early Precision QT methodology optimizes data collection, transmission, and analysis. Waveform interference is minimized by consistently enforcing electronics restrictions within the clinic when ECG data is being collected. In addition, medical procedures are meticulously scheduled around extraction time points in order to make EPQT/TQT trials successful.
If you would like to receive more information regarding how our CRO can be leveraged for your upcoming cardiac assessment study, please contact us.
Thorough QT/QTc and Early Precision QT Study Design
The design of the study performed will first be determined by whether a thorough QT/QTc study is being performed, or a sponsor is choosing to incorporate early QT assessments into the design of a FIH clinical trial.
Thorough QT/QTc
Typically, a thorough QT/QTc study is performed as a double-blind, randomized, crossover study in normal healthy volunteers. This trial is either conducted at the end of Phase 2 or in parallel with Phase 3 studies and typically includes 40-60 subjects (1).
In the crossover design, trial volunteers are randomized to 1 of 4 treatment groups and receive each of the study treatments (or placebo) in a random sequence, with washout periods in between. Alternative study designs may be considered depending on the pharmacokinetics of the investigational product; however, the treatment groups are typically as follows:
- Treatment 1: Placebo
- Treatment 2: Positive control
- Treatment 3: Therapeutic dose of investigational product
- Treatment 4: Supratherapeutic dose of investigational product
As stated in the FDA guidance, “this study has a critical role in determining the intensity of ECG data collection during later stages of drug development (2).” A “negative” study will allow for the collection of on-therapy ECGs in accordance with current practices to constitute sufficient evaluation during later stages of clinical development. A “positive” study will almost always require that expanded ECG safety evaluation should be performed during late-stage clinical trials.
Early Precision QT
In this design, early precision QT assessments are incorporated into the design of a FIH, single-ascending dose and multiple ascending dose (SAD/MAD) trial. This approach differs from more common cardiac safety assessments performed in Phase 1, which is typically based on the principal investigator’s read of 12-lead ECGs. The early precision QT methodology leverages both concentration effect modeling, as well as high precision analysis of 10x more data than conventional measurement methods (1).
It is advised that at minimum, six to nine subjects at sufficiently high dose levels (plasma levels) are evaluated using the EPQT approach. Adding doses to achieve plasma concentrations above targeted therapeutic levels is helpful. Another item to consider is the number of subjects receiving placebo, typically pooled across several dose groups. A minimum number of 8 subjects on placebo is ideal. According to Clario, “it seems prudent to use at least 4 dose groups with 6/2 on active/placebo and with two of the doses higher than what is believed to be the therapeutic dose (1).”
Thorough QT/QTc and Early Precision QT Study Design
The design of the study performed will first be determined by whether a thorough QT/QTc study is being performed, or a sponsor is choosing to incorporate early QT assessments into the design of a FIH clinical trial.
Thorough QT/QTc
Typically, a thorough QT/QTc study is performed as a double-blind, randomized, crossover study in normal healthy volunteers. This trial is either conducted at the end of Phase 2 or in parallel with Phase 3 studies and typically includes 40-60 subjects.(1)
In the crossover design, trial volunteers are randomized to 1 of 4 treatment groups and receive each of the study treatments (or placebo) in a random sequence, with washout periods in between. Alternative study designs may be considered depending on the pharmacokinetics of the investigational product; however, the treatment groups are typically as follows:
Treatment 1: Placebo
Treatment 2: Positive control
Treatment 3: Therapeutic dose of investigational product
Treatment 4: Supratherapeutic dose of investigational product
As stated in the FDA guidance, “this study has a critical role in determining the intensity of ECG data collection during later stages of drug development.”(2) A “negative” study will allow for the collection of on-therapy ECGs in accordance with current practices to constitute sufficient evaluation during later stages of clinical development. A “positive” study will almost always require that expanded ECG safety evaluation should be performed during late-stage clinical trials.
- Treatment 1: Placebo
- Treatment 2: Positive control
- Treatment 3: Therapeutic dose of investigational product
- Treatment 4: Supratherapeutic dose of investigational product
Typically, a thorough QT/QTc study is performed as a double-blind, randomized, crossover study in normal healthy volunteers. This trial is typically conducted at the end of Phase 2 or in parallel with Phase 3 studies and includes 40-60 subjects.(1)
In the crossover design, trial volunteers are randomized to 1 of 4 treatment groups and receive each of the study treatments (or placebo) in a random sequence, with washout periods in between. Alternative study designs may be considered depending on the pharmacokinetics of the investigational product; however, the treatment groups are typically as follows:
Treatment 1: Placebo
Treatment 2: Positive control
Treatment 3: Therapeutic dose of investigational product
Treatment 4: Supratherapeutic dose of investigational product
As stated in the FDA guidance, “this study has a critical role in determining the intensity of ECG data collection during later stages of drug development.”(2) A “negative” study will allow for the collection of on-therapy ECGs in accordance with current practices to constitute sufficient evaluation during later stages of clinical development. A “positive” study will almost always require that expanded ECG safety evaluation should be performed during late-stage clinical trials.
Early Precision QT
In this design, early precision QT assessments are incorporated into the design of a FIH, single-ascending dose and multiple ascending dose (SAD/MAD) trial. This approach differs from more common cardiac safety assessments performed in Phase 1, which is typically based on the principal investigator’s read of 12-lead ECGs. The early precision QT methodology leverages both concentration effect modeling, as well as high precision analysis of 10x more data than conventional measurement methods.(1)
It is advised that at minimum, six to nine subjects at sufficiently high dose levels (plasma levels) are evaluated using the EPQT approach. Adding doses to achieve plasma concentrations above targeted therapeutic levels is helpful. Another item to consider is the number of subjects receiving placebo, typically pooled across several dose groups. A minimum number of 8 subjects on placebo is ideal. According to Clario, “it seems prudent to use at least 4 dose groups with 6/2 on active/placebo and with two of the doses higher than what is believed to be the therapeutic dose.”(1)
How DVCR Obtains High-Quality Data for Cardiac Assessment Studies
In either study design, trials need to be supported by market-leading technology and equipment. By leveraging the Hillrom Surveyor™, DVCR’s advanced cardiovascular life support staff are able to view the status of all Holter monitors from either a central station or locally from the S4’s LCD screens. This allows for our team to quickly resolve any issues with low batteries or poor lead connections quickly and efficiently.
The monitoring parameters and values are customizable based upon the requirements of each unique protocol. Once the monitoring session is complete, the data is archived to a dedicated server using Surveyor™ Reviewer software. From there it can be sent or uploaded to the designated destination for further review and examination. The above Surveyor™ system allows the clinical team to more effectively collect and transmit data for improved quality and efficiency when conducting cardiac assessment trials.
In order to ensure that the best data quality is obtained, each study volunteer is prepped and patched with great attention to detail. This includes the removal of body hair, cleaning of electrode application site, and placement using appropriate anatomical landmarks. To minimize the risk of electrical interference, all cellular phones and non-essential electronic devices are powered off during procedures.
At designated extraction timepoints, DVCR staff instruct study volunteers to remain still and communicate when they are free to move about the clinic. During these extraction periods, staff verify via the Holter monitor that all leads are attached, and the device is recording appropriately.
Benefits of Incorporating QT Assessments in Phase I Trials

Cost Savings: By obtaining the necessary cardiac safety data earlier on in the clinical development process, sponsors can in many cases forego the need to perform a thorough QT study which could result in millions of dollars of savings overall.
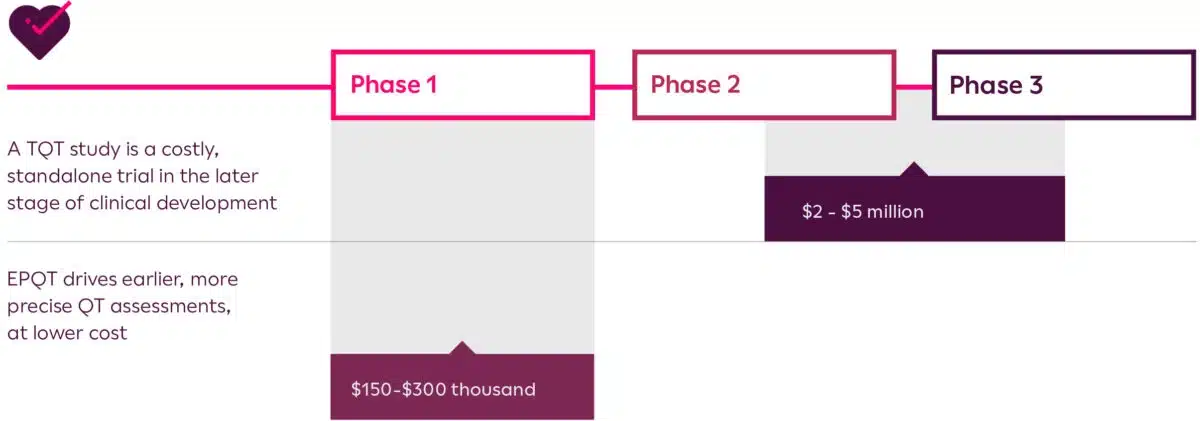
Graphic provided by Clario (4)

Time Savings: By removing a full TQT study from an asset’s clinical development program, drug development companies can potentially introduce their asset to the marketplace sooner.

Pipeline Prioritization and De-Risking: It would be unfortunate for a potential new and safe therapy to be labeled as “QT-prolonging” due to the high incidence of inconclusive positive and false positive results which are found in traditional approaches to cardiac safety assessments. By conducting more accurate early QT studies, these risks can be mitigated. Furthermore, by collecting cardiac safety data earlier in an asset’s development program, sponsors can prioritize those clinical programs with stronger safety profiles over those with potential cardiac safety issues in late-stage trials (1).
References
1. Darpo, B. (2022, April). The Early Precision QT Approach: Driving earlier assessments of cardiac safety and supporting regulatory change. Endpoint Technology Services for Clinical Trial Management | Clario. Retrieved April 2023, from https://clario.com/wp-content/uploads/2022/04/Clario_White-paper_EPQT-24MAR2022.pdf
2. Food and Drug Administration. (2012, October). Guidance for Industry: E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. Retrieved April 2023, from https://www.fda.gov/media/71372/download
3. Darpo, B., Borin, M., Ferber, G., Galluppi, G.R., Hopkins, S.C., Landry, I., Lo, A., Rege, B., Reyderman, L., Sun, L., Watanabe, T., Xue, H. and Yasuda, S. (2022), ECG Evaluation as Part of the Clinical Pharmacology Strategy in the Development of New Drugs: A Review of Current Practices and Opportunities Based on Five Case Studies. J Clin Pharm, 62: 1480-1500. https://doi.org/10.1002/jcph.2095
4. Clario. (2021, March). Early Precision QT | Clario. Endpoint Technology Services for Clinical Trial Management | Clario. Retrieved April 2023, from https://clario.com/resources/downloads/early-precision-qt/

