Hepatic Impairment Studies
The effect of hepatic disease on the absorption and disposition of drugs (pharmacokinetics) as well as their safety and efficacy (pharmacodynamics) is well-documented across biomedical literature (1). As the liver is involved in the clearance of multiple drugs via oxidative and conjugative metabolic pathways and through biliary excretion, hepatic impairment can negatively impact the safety of a drug by accumulation of the drug or its metabolites in toxic concentrations or impact the efficacy of the drug which would necessitate a dose adjustment in this patient population (2). Therefore, it is imperative to evaluate the impact of varying levels of hepatic impairment on the pharmacokinetics (PK) of a new drug and its metabolites in order to define a safe and efficacious dose for these patients.
If hepatic metabolism and/or excretion accounts for a substantial portion of the elimination of a parent drug or its active metabolites (>20% of the absorbed drug), the FDA recommends a hepatic impairment study in order to adequately develop the recommendations for dose and dosing interval (1). If there is a narrow therapeutic range for the drug or for drugs with unknown metabolism, the guidance recommends a study also be performed even if less than 20% of the drug is hepatically cleared (1,2).
Reach Out Today
Learn how DVCR can support your hepatic impairment clinical trials.
Renal and Hepatic Impairment Studies Fact Sheet
UPCOMING WEBINAR
Effective Study Design and Execution of Renal and Hepatic Impairment Trials
Friday, November 8, 2024 | 2pm EST (11am PST)
The effect of hepatic disease on the absorption and disposition of drugs (pharmacokinetics) as well as their safety and efficacy (pharmacodynamics) is well-documented across biomedical literature (1). As the liver is involved in the clearance of multiple drugs via oxidative and conjugative metabolic pathways and through biliary excretion, hepatic impairment can negatively impact the safety of a drug by accumulation of the drug or its metabolites in toxic concentrations or impact the efficacy of the drug which would necessitate a dose adjustment in this patient population (2). Therefore, it is imperative to evaluate the impact of varying levels of hepatic impairment on the pharmacokinetics (PK) of a new drug and its metabolites in order to define a safe and efficacious dose for these patients.
If hepatic metabolism and/or excretion accounts for a substantial portion of the elimination of a parent drug or its active metabolites (>20% of the absorbed drug), the FDA recommends a hepatic impairment study in order to adequately develop the recommendations for dose and dosing interval (1). If there is a narrow therapeutic range for the drug or for drugs with unknown metabolism, the guidance recommends a study also be performed even if less than 20% of the drug is hepatically cleared (1,2).
Reach Out Today
Learn how DVCR can support your hepatic impairment clinical trials.
Renal & Hepatic Impairment Studies Fact Sheet
Renal & Hepatic Impairment Studies Fact Sheet
UPCOMING WEBINAR
Effective Study Design and Execution of Renal and Hepatic Impairment Trials
Friday, November 8, 2024
UPCOMING WEBINAR
Effective Study Design and Execution of Renal and Hepatic Impairment Trials
Friday, November 8, 2024 | 2pm EST (11am PST)
Hepatic Impairment Study Design
Hepatic impairment studies can be designed as either a single-dose or multiple-dose study with PK assessment of the parent drug and any active metabolites. A single-dose study may be appropriate for cases where prior evidence states that multiple-dose PK is accurately predicted by single-dose data for both the parent drug and active metabolites. The multiple-dose study is more appropriate when the drug or an active metabolite is known to exhibit nonlinear or time-dependent PK.
For both studies, the planned clinical dose is typically recommended, but a reduced dose may be considered if there is a concern about drug toxicity in hepatically impaired patients with increased blood levels. The route of drug administration should also be the same as that intended for clinical use. For drugs where more than one route of administration is proposed, the hepatic impairment study should use the route that provides the most information regarding the impact of hepatic impairment on the drug’s elimination (1,2).
Subjects should have stable hepatic impairment, “defined as no clinically significant change in disease status within a certain time (at the discretion of the investigator) before screening (2).” Because subjects with hepatic impairment often have comorbidities such as diabetes and hypertension, investigators may allow these populations or those with concomitant medications (whose condition is stable) to be enrolled.
Categories of hepatic impairment are most commonly measured by the Child-Pugh score, which employs five clinical measures of liver disease. Each measure is scored between 1 and 3, with 3 indicating the most severe level of impairment (3). The corresponding scores for all measures are added to classify the patients into mild (score of 5-6), moderate (score of 7-9) or severe (score of 10-15). The clinical measures of liver disease and their scales are outlined in the following table:
Child-Pugh criteria for staging liver impairment
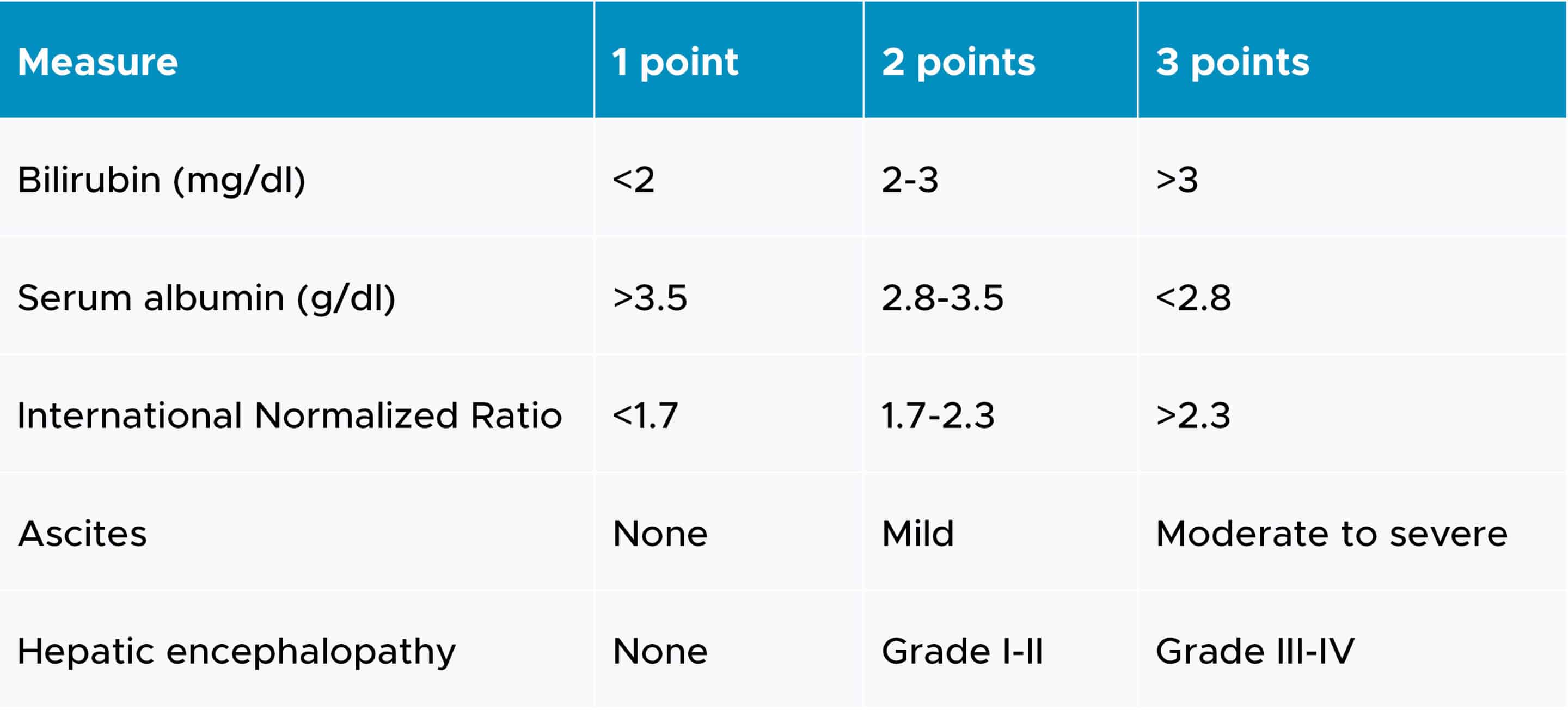
Both full and reduced study designs have been utilized. A full study design requires the enrollment of subjects in all three Child-Pugh categories, as well as subjects with normal hepatic function. At least six participants in each arm should be evaluated for this study design to provide valuable data (1).
In the reduced study design, the FDA suggests comparing the PK of the drug in subjects with moderate level hepatic impairment to subjects with normal liver functions and enrolling at least eight subjects in both groups. The results from this design can guide dosing instructions or the need for further assessment in patients with mild or severe levels of impairment (2).
Control subjects should be matched to the hepatically impaired subjects with respect to age, gender and weight. Additionally, other factors such as smoking, alcohol intake, concomitant medications and ethnicity/race should be considered as these factors can potentially affect the drug’s PK (2).
DVCR Model for Hepatic Impairment Studies
DVCR has launched a unique and site-centric approach to successfully manage hepatic impairment studies. We have partnered with strategic sites and Subject Matter Experts that have conducted more than 150 combined hepatic impairment studies over the last five years. These sites have access to mild, moderate and severe patients as well as healthy controls and collectively provide access to over 300 beds for overnight confinement.
DVCR’s CRO services coupled with the collaborative approach with these strategic sites ensures patient enrollment and critical milestones are met for hepatic impairment studies.
DVCR assigns tenured project managers who understand the challenges of complex clinical pharmacology studies and have extensive experience managing multi-site trials. Lastly, our model expedites site payments to de-risk study delays and ensures the sites remain engaged and committed to the study.
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Reach Out Today
If you would like to learn more about DVCR and how our CRO services can support your upcoming hepatic impairment clinical trial, please contact us.
References
1 U.S. Food and Drug Administration, Center for Drug Evaluation and Research, & Center for Biologics Evaluation and Research. (2003). (rep.). Pharmacokinetics in Patients with Impaired Hepatic Function: Study Design, Data Analysis, and Impact on Dosing and Labeling. Retrieved October 24, 2023, from https://www.fda.gov/media/71311/download.
2 Ravenstijn, P., Chetty, M., Manchandani, P., Elmeliegy, M., Qosa, H., & Younis, I. (2022). Design and conduct considerations for studies in patients with hepatic impairment. Clinical and Translational Science, 16(1), 50–61. https://doi.org/10.1111/cts.13428
3 Wikimedia Foundation. (2023, August 12). Child–pugh score. Wikipedia. https://en.wikipedia.org/wiki/Child%E2%80%93Pugh_score
