Renal Impairment Studies
Many drugs are eliminated from the body through a combination of some or all of the following organs: small intestine, liver and kidneys. For those drugs cleared primarily via renal excretion, it is known that impaired renal function will typically alter the drug’s pharmacokinetics (PK), as well as potentially impact the drug’s absorption, bioavailability, tissue distribution and plasma protein binding (1,2). Therefore, in order to prevent a loss of therapeutic effect and/or increase in safety risks, a different dosing regimen between patients with normal renal function and those with impaired renal function should be given consideration.
To inform the dosing recommendations, a dedicated renal impairment study is generally recommended. A renal impairment study aims to study the effect of mild, moderate and severe renal impairment on the PK of a drug or its active metabolites.
The team at Dr. Vince Clinical Research understands the unique challenges associated with PK renal impairment studies and provides a wide range of services to conduct them efficiently and as expeditiously as possible.
Our Model for Renal Impairment Studies
In order to offer the best solution possible, DVCR has prioritized its relationship with a network of strategically selected sites that have extensive experience conducting renal impairment studies. Our team has built trusted relationships with these sites, and our site-centric approach ensures sites are highly engaged and collaborative. Not only do these sites have access to the necessary patient populations as referenced in the FDA guidance, but they are also overseen by well-known Subject Matter Experts in this space who understand how to operationalize each study.
Our model for successfully conducting renal impairment studies involves:

Establishing governance charters, clear communication and escalation pathways through a proactive approach to site partnerships

Expediting site payments to de-risk study delays and encourage site engagement and study prioritization

Assigning only tenured project managers who have experience overseeing multisite studies and understand the nuances of complex clinical pharmacology studies
Our approach positively impacts study timelines, patient recruitment and data quality. For these studies, DVCR actively manages the sites, ensuring patient recruitment and other deliverables are met on time. Our CRO services, including data management, biostatistics and programming, monitoring and medical writing provide a seamless solution for our biopharma clients.
Site Network Overview
Reach Out Today

Robust network of sites across North America

Our site network has conducted over 130 PK renal impairment studies over the past five years

Access to over 350 beds for overnight confinement

Access to patients with mild, moderate and severe renal impairment as well as patients with End Stage Renal Disease (ESRD) on hemodialysis
Site Network Overview

Our site network has conducted over 130 PK renal impairment studies over the past five years

Access to over 350 beds for overnight confinement

Robust network of sites across North America

Access to patients with mild, moderate and severe renal impairment as well as patients with End Stage Renal Disease (ESRD) on hemodialysis

Reach Out Today
PK Renal Impairment Study Design
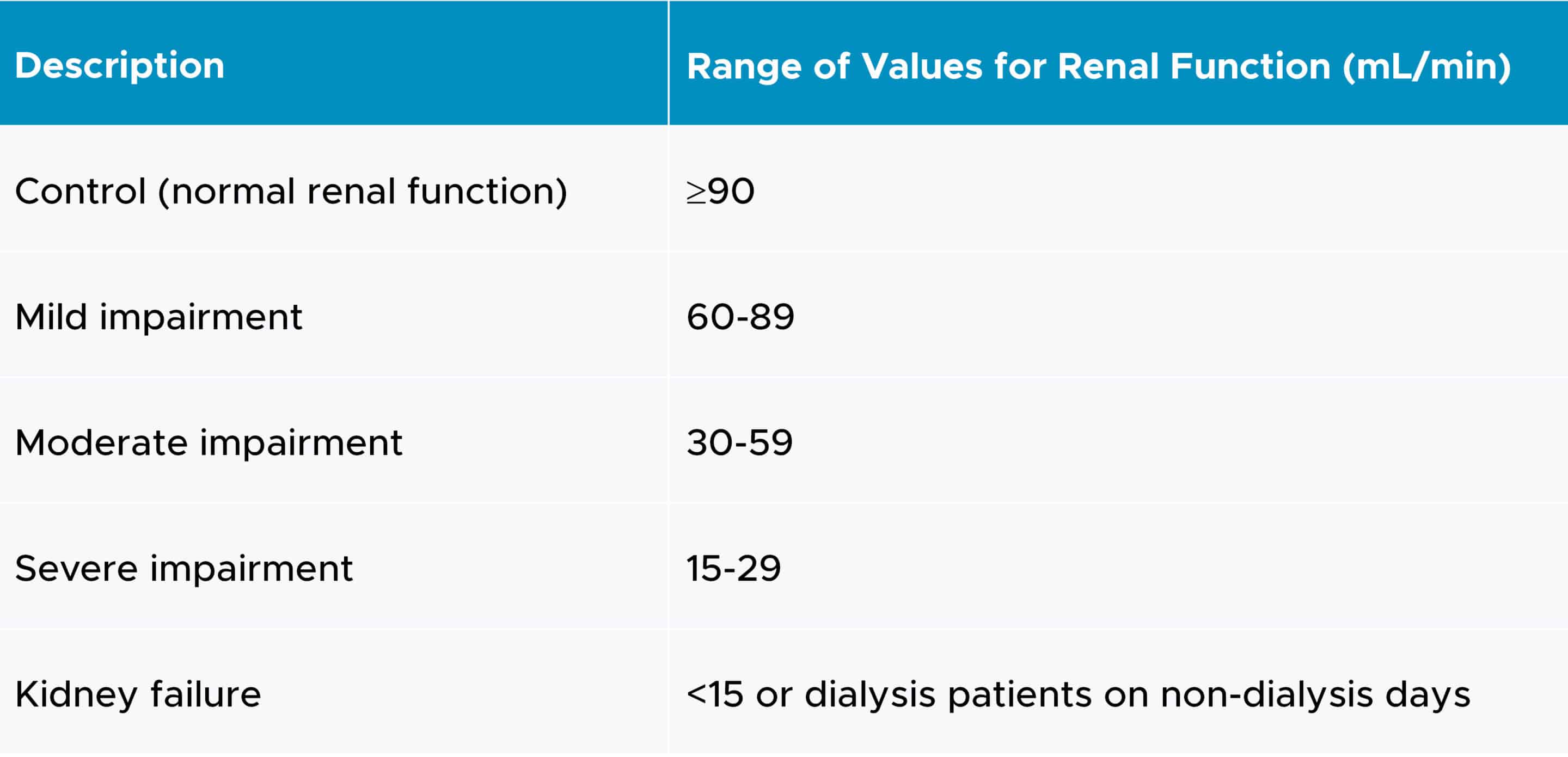
Typically, a single-dose study is sufficient to accurately describe the PK of the drug and its active metabolites. In most single-dose studies, the same dose can be administered to all patients regardless of renal function because the peak concentration of the drug is not substantially impacted by renal function. A parallel group study design is used, and each group should enroll between 6-8 subjects (2).
For the control group, consideration should be given to matching various demographic factors such as age, weight, sex and ethnicity. A 1:1 matching strategy can be utilized, wherein participants in each impaired group are matched with individuals in the control group (2).
1 U.S. Food and Drug Administration, & Center for Drug Evaluation and Research (CDER). (2020). (rep.). Pharmacokinetics in Patients with Impaired Renal Function — Study Design, Data Analysis, and Impact on Dosing. U.S. Food and Drug Administration. Retrieved October 24, 2023, from https://www.fda.gov/media/78573/download.
2 Ravenstijn, P., Chetty, M., & Manchandani, P. (2021). Design and conduct considerations for studies in patients with impaired renal function. Clinical and Translational Science, 14(5), 1689–1704. https://doi.org/10.1111/cts.13061
PK Renal Impairment Study Design
Typically, a single-dose study is sufficient to accurately describe the PK of the drug and its active metabolites. In most single-dose studies, the same dose can be administered to all patients regardless of renal function because the peak concentration of the drug is not substantially impacted by renal function. A parallel group study design is used, and each group should enroll between 6-8 subjects (2).

For the control group, consideration should be given to matching various demographic factors such as age, weight, sex and ethnicity. A 1:1 matching strategy can be utilized, wherein participants in each impaired group are matched with individuals in the control group (2).
1 U.S. Food and Drug Administration, & Center for Drug Evaluation and Research (CDER). (2020). (rep.). Pharmacokinetics in Patients with Impaired Renal Function — Study Design, Data Analysis, and Impact on Dosing. U.S. Food and Drug Administration. Retrieved October 24, 2023, from https://www.fda.gov/media/78573/download.
2 Ravenstijn, P., Chetty, M., & Manchandani, P. (2021). Design and conduct considerations for studies in patients with impaired renal function. Clinical and Translational Science, 14(5), 1689–1704. https://doi.org/10.1111/cts.13061
