Human Abuse Potential
The abuse of prescription drugs, such as opioids, stimulants, and central nervous system (CNS) depressants has become a prominent public health concern in the United States and around the world. Due to the increasing rate of prescription drug abuse, regulatory requirements for the abuse potential assessment of CNS-active drugs have become increasingly stringent, particularly in the US (i.e., FDA). The process by which new molecular entities (NMEs) are evaluated by sponsors and reviewed by regulators has evolved over time, with many biopharmaceutical companies now being required to conduct a human abuse potential/human abuse liability (HAP/HAL) study.
Human abuse potential studies are typically conducted as single-dose, double-blind, randomized, placebo and active-controlled, crossover studies that evaluate the subjective effects of the investigational drug in “recreational” (non-dependent) drug users. The comparison with placebo is used to make conclusions about ‘absolute’ abuse potential (i.e., determining whether the drug has any intrinsic abuse potential). The comparison with the active comparator, a scheduled drug with known abuse liability, is used to make conclusions about ‘relative’ abuse potential. The data from HAP studies is important towards developing abuse-related drug product labeling and in determining whether a NME will be scheduled under the DEA’s Controlled Substances Act.
Over the last 20 years, the team at Dr. Vince Clinical Research has developed deep experience in the design, planning, and conduct of HAP studies, taking into consideration the unique subject population, critical endpoints, and operational complexities which are crucial towards successful study execution.
Contact Us to Learn More About our Human Abuse Potential Capabilities
Summary of Human Abuse Potential Webinar
Human Abuse Potential: Participant Safety
Statistical Analysis in Human Abuse Potential Studies
The abuse of prescription drugs, such as opioids, stimulants, and central nervous system (CNS) depressants has become a prominent public health concern in the United States and around the world. Due to the increasing rate of prescription drug abuse, regulatory requirements for the abuse potential assessment of CNS-active drugs have become increasingly stringent, particularly in the US (i.e., FDA). The process by which new molecular entities (NMEs) are evaluated by sponsors and reviewed by regulators has evolved over time, with many biopharmaceutical companies now being required to conduct a human abuse potential/human abuse liability (HAP/HAL) study.
Human abuse potential studies are typically conducted as single-dose, double-blind, randomized, placebo and active-controlled, crossover studies that evaluate the subjective effects of the investigational drug in “recreational” (non-dependent) drug users. The comparison with placebo is used to make conclusions about ‘absolute’ abuse potential (i.e., determining whether the drug has any intrinsic abuse potential). The comparison with the active comparator, a scheduled drug with known abuse liability, is used to make conclusions about ‘relative’ abuse potential. The data from HAP studies is important towards developing abuse-related drug product labeling and in determining whether a NME will be scheduled under the DEA’s Controlled Substances Act.
Over the last 20 years, the team at Dr. Vince Clinical Research has developed deep experience in the design, planning, and conduct of HAP studies, taking into consideration the unique subject population, critical endpoints, and operational complexities which are crucial towards successful study execution.
Contact Us to Learn More About our Human Abuse Potential Capabilities
Summary of Human Abuse Potential Webinar
Human Abuse Potential: Participant Safety
Statistical Analysis in Human Abuse Potential Studies
DVCR’s Human Abuse Potential Experience

Investigator and Scientific team have been involved in the conduct of over 75 HAP studies
Participated in government-funded projects

In-house VAS Raters with deep understanding of cognitive scales

Dedicated Safety team with decades of experience (ACLS certified)

Leadership Team averaging a decade of experience in early clinical development and participation in nearly 10 HAP studies each

3 full-time pharmacists with over 50 years’ combined research experience:
– Experience with over-encapsulation and blinding investigational products
– Worked with multiple classes of medications including opioids, stimulants, hallucinogens, hypnotics, depressants, sedatives, dissociatives, and cannabinoids
– Multiple routes of administration including injectable formulations, nasal insufflation, powder in capsule (PIC) and others

Specialized protocol and CSR writing services with experts in Human Abuse Liability
Press Release covering recent Human Abuse Potential study
Clinical Trials.gov: NCT05571345
DVCR’s Human Abuse Potential Experience

Investigator and Scientific team have been involved in the conduct of over 75 HAP studies
Participated in government-funded projects

3 full-time pharmacists with over 50 years’ combined research experience:
– Experience with over-encapsulation and blinding investigational products
– Worked with multiple classes of medications including opioids, stimulants, hallucinogens, hypnotics, depressants, sedatives, dissociatives, and cannabinoids
– Multiple routes of administration including injectable formulations, nasal insufflation, powder in capsule (PIC) and others

Leadership Team averaging a decade of experience in early clinical development and participation in nearly 10 HAP studies each

Dedicated Safety team with decades of experience (ACLS certified)

In-house VAS Raters with deep understanding of cognitive scales

Specialized protocol and CSR writing services with experts in Human Abuse Liability
Press Release covering recent Human Abuse Potential study
Clinical Trials.gov: NCT05571345
Clinical Pharmacology Unit: Purpose-Built for HAP Studies

Multiple aspects of our Phase 1 unit were designed with the goal of conducting successful Human Abuse Potential studies
Multiple aspects of our Phase 1 unit were designed with the goal of conducting successful Human Abuse Potential studies

Five dedicated rooms for pupillometry

90 beds for overnight confinement, including 8 private suites for individual subject domiciling if needed

Round-the-clock video surveillance in subject areas, with crash carts and panic buttons strategically located throughout the facility

Five dedicated rooms for pupillometry

90 beds for overnight confinement, including 8 private suites for individual subject domiciling if needed

Round-the-clock video surveillance in subject areas, with crash carts and panic buttons strategically located throughout the facility
In order to conduct Human Abuse Potential studies, comprehensive pharmacy capabilities are required. DVCR’s pharmacy team has over 50 years’ experience in clinical pharmacology studies, including extensive expertise with the handling and manipulation of study drugs for various routes of administration in HAP/HAL clinical trials. These include, but are not limited to injectable formulations (e.g., IV, IM), nasal insufflation, and powder in capsule (PIC).
For those studies requiring blinding of the reference therapy, our team utilizes over-encapsulation as an effective strategy in maintaining the blind. Not only does our team have the ability to provide accurate individual doses for trial volunteers, but our state-of-the-art cGMP pharmacy includes a USP-795 compliant non-sterile compounding room. This room features independent narrow spectrum 570nm amber LED lights for handling photosensitive API study drug. In addition, any API powder requiring weighing for batch preparation will be performed using our analytical balance. The balance is housed within a Type B PowderSafe™ enclosure outfitted with a HEPA filter and resides on an anti-vibration table to improve balance performance.
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Reach Out Today
If you would like to receive more information regarding how our CRO can be leveraged for your upcoming abuse potential trial, please contact us.
In order to conduct Human Abuse Potential studies, comprehensive pharmacy capabilities are required. DVCR’s pharmacy team has over 50 years’ experience in clinical pharmacology studies, including extensive expertise with the handling and manipulation of study drugs for various routes of administration in HAP/HAL clinical trials. These include, but are not limited to injectable formulations (e.g., IV, IM), nasal insufflation, and powder in capsule (PIC).
For those studies requiring blinding of the reference therapy, our team utilizes over-encapsulation as an effective strategy in maintaining the blind. Not only does our team have the ability to provide accurate individual doses for trial volunteers, but our state-of-the-art cGMP pharmacy includes a USP-795 compliant non-sterile compounding room. This room features independent narrow spectrum 570nm amber LED lights for handling photosensitive API study drug. In addition, any API powder requiring weighing for batch preparation will be performed using our analytical balance. The balance is housed within a Type B PowderSafe™ enclosure outfitted with a HEPA filter and resides on an anti-vibration table to improve balance performance.

For those studies requiring blinding of the reference therapy, our team utilizes over-encapsulation as an effective strategy in maintaining the blind. Not only does our team have the ability to provide accurate individual doses for trial volunteers, but our state-of-the-art cGMP pharmacy includes a USP-795 compliant non-sterile compounding room. This room features independent narrow spectrum 570nm amber LED lights for handling photosensitive API study drug. In addition, any API powder requiring weighing for batch preparation will be performed using our analytical balance. The balance is housed within a Type B PowderSafe™ enclosure outfitted with a HEPA filter and resides on an anti-vibration table to improve balance performance.
Reach Out Today
If you would like to receive more information regarding how our CRO can be leveraged for your upcoming abuse potential trial, please contact us.
Human Abuse Liability Study Design
Typically, Human Abuse Liability studies are organized into four phases: Screening, Qualification, Treatment, and Follow-Up. Investigational products are administered in both the Qualification and Treatment phases.

Qualification Phase
During the Qualification Phase, trial volunteers are evaluated on their ability to distinguish between a placebo and a scheduled drug with known abuse liability, referred to as the “positive control.” Only subjects who report drug liking in response to the positive control will be selected to move on to the Treatment phase.

Treatment Phase
In most cases, the Treatment Phase is designed as a randomized, double-blind, double-dummy, placebo- and active-controlled, crossover study. During this phase subjects will receive placebo, one or two doses of the positive control and three doses of the investigational product. Each study treatment should be administered once to each subject using a repeated Williams squared design.
Because the sample size of human abuse potential studies is relatively small, study powering is of special concern. In order to determine the necessary sample size, a power analysis should be conducted, and justification provided of the power level that was used in the calculation.
HAP studies will typically include outcome measures such as safety and physiological measures, pharmacokinetic data, abuse-related adverse events, as well as subjective measures taken from trial volunteers.
Subjective Measures and Visual Analog Scales
HAP studies are unique in that the endpoint is focused on the subjective ratings of study participants (e.g., at the moment drug liking, also known as Emax). Trial volunteers with self-reported drug use are queried concerning the effects of the investigational product being administered. Therefore, trial volunteers must receive extensive training on the importance of the assessments without coaching them into giving any specific responses. Highly trained staff are required to strike this balance.
To ensure the best quality data is produced, DVCR has a full-time Ratings Manager responsible for protocol review, staff oversight, and participant training. DVCR has heavily invested in materials, systems, and personnel to ensure these complex studies are executed carefully.
Our team leverages Cambridge Cognition’s Clinical Trial Information System for Abuse Liability (CTIS-AL) which enables configuration of questionnaires and visual analog scales (VAS) to ensure outcome measures meet the exact objectives of the trial. Scales have exact selection capability on unipolar and bipolar scales for high precision and sensitive detection of change over time. This system allows for the following
- Computerized, paperless administration and data collection
- Configurable questionnaires and visual analog scales
- Immediate data storage and real-time data access
- Electronic management of volunteer visit schedules
- Dedicated operational and scientific support
Cambridge Cognition is unique in its graphical reporting capability, which supports sponsors in determining whether to progress a participant from qualification to the next phase. Furthermore, the Company has significant experience in supporting Abuse Liability studies, having supported over 120 of these types of studies, and produced data that has been submitted to regulatory agencies (1).
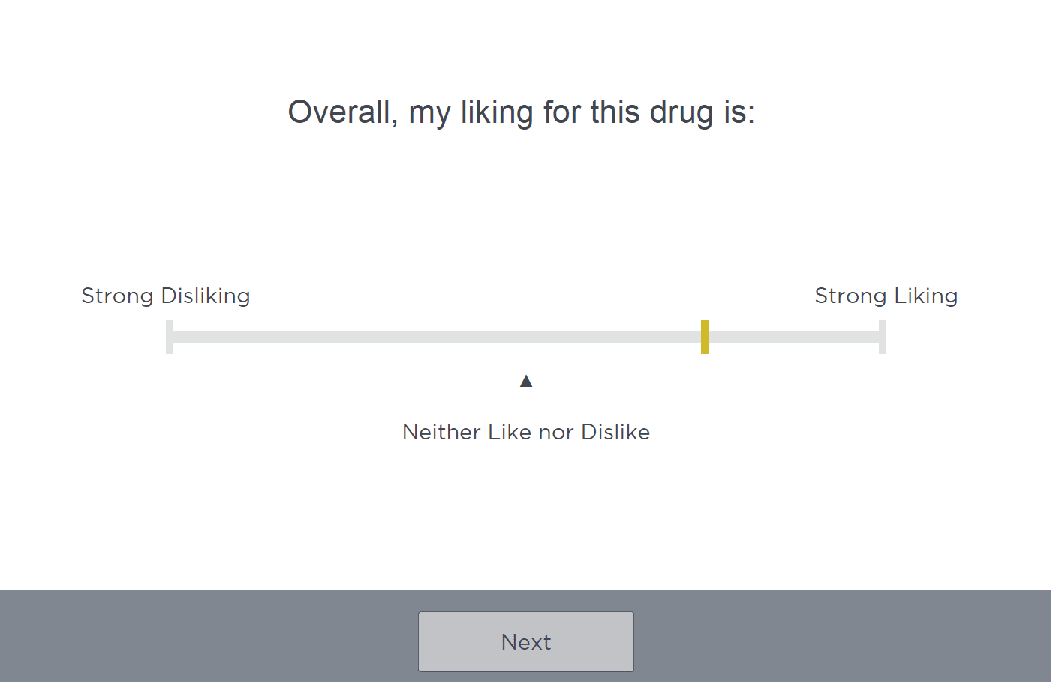

Our team leverages Cambridge Cognition’s Clinical Trial Information System for Abuse Liability (CTIS-AL) which enables configuration of questionnaires and visual analog scales (VAS) to ensure outcome measures meet the exact objectives of the trial. Scales have exact selection capability on unipolar and bipolar scales for high precision and sensitive detection of change over time. This system allows for the following
- Computerized, paperless administration and data collection
- Configurable questionnaires and visual analog scales
- Immediate data storage and real-time data access
- Electronic management of volunteer visit schedules
- Dedicated operational and scientific support
Cambridge Cognition is unique in its graphical reporting capability, which supports sponsors in determining whether to progress a participant from qualification to the next phase. Furthermore, the Company has significant experience in supporting Abuse Liability studies, having supported over 120 of these types of studies, and produced data that has been submitted to regulatory agencies (1).
Common Measures for HAL Studies

At the Moment Drug Liking

Drug effects (positive and negative)

Drug similarity

Subjective drug value

Overall drug liking

Likelihood to take drug again
DVCR works closely with its partners to develop a plan to capture subjective volunteer data using the most advanced technology available and ensure study endpoints can be successfully measured.
Volunteer Recruitment and Retention for HAL Studies
Targeted volunteer recruitment is a critical element in the success of Human Abuse Liability studies. HAP studies are to be conducted in experienced drug users who have experience using drugs which are in the same pharmacological class as the investigational product. The use of drug-naïve subjects has not been validated scientifically as being able to provide accurate information on the abuse potential of a drug.
Therefore, having a robust database of potential study volunteers that have experience abusing the drug class and are able to meaningfully discriminate between the selected positive control and the placebo is essential in the successful operational conduct of these complex clinical trials. Furthermore, it is imperative that Sponsors plan accordingly to account for potential volunteer dropouts in order to ensure that the study is adequately powered.
DVCR maintains an active database of substance users that have experience abusing various drug classes. The 2017 FDA Guidance Assessment of Abuse Potential of Drugs notes that “Sponsors should include a fair representation of races and sexes as participants in clinical trials so that clinically significant differences in response can be detected. The FDA Guideline for the Study and Evaluation of Gender Differences in the Clinical Evaluation of Drugs (1993) should be followed (2).” DVCR is committed to both speed and diversity in recruitment and facilitates these goals with luxurious amenities for trial volunteers to enjoy
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Regulatory Considerations for Human Abuse Potential Studies
FDA’s guidance indicates that CNS-active drugs will require an assessment of abuse potential and may be subject to control under the Controlled Substances Act (CSA). The requirement for a human abuse potential study is based on an evaluation of data from nonclinical and clinical studies, such as abuse-related adverse events in healthy subjects and patients.
Prior to conducting a human abuse potential study, it is recommended that Sponsors submit both a protocol and statistical analysis plan to the Controlled Substance Staff (CSS), located in CDER’s Office of the Center Director. This body has the central role in CDER in advising sponsors regarding the abuse potential assessment of an NME. The design of each human abuse potential study has complexities related to the characteristics of the NME that is being evaluated and therefore requires product-specific consulting.
References
1 Drug abuse liability. Cambridge Cognition. (n.d.). Retrieved April 28, 2023, from https://www.cambridgecognition.com/products/drug-development/develop-new-treatments/abuse-liability
2 U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). (2017, January). Assessment of abuse potential of drugs guidance for industry. Retrieved April 28, 2023, from https://www.fda.gov/media/116739/download

